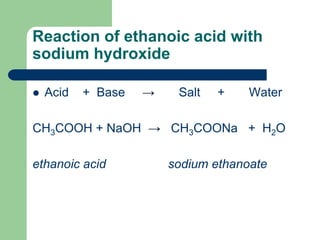
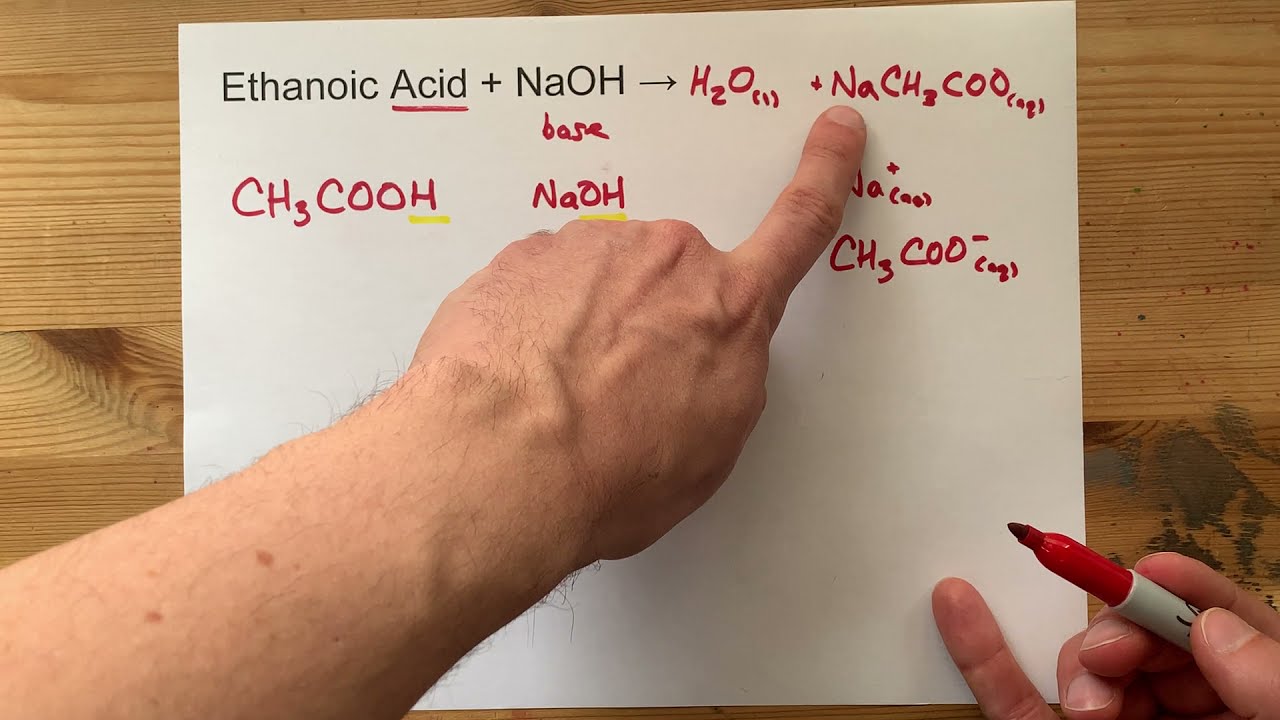
Ethanoic Acid and Sodium Hydroxide
The health hazards of potassium hydroxide are similar to those of the other strong alkalies such as sodium. Oxygen nitrogen and the rare gases.
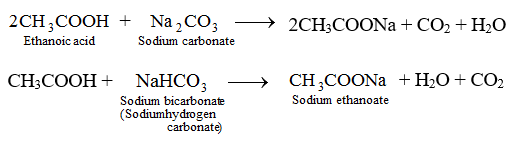
Physical Amd Chemical Properties Of Ethanoic Aacid Chemistry Knowledgeuniverseonline Com
NaOH HClO 4 NaClO 4 H 2 O.
. ChlorineVII oxide itself also reacts with sodium hydroxide solution to give the same. Factory Laboratory CAS 64-19-7 Ethanoic Acid Chemical Reagent Acetic Acid FOB Price. Bases are usually found to have a bitter taste and feel slippery soap is a good example.
Identify any buffer regions on your titration curve. In other words the concentration of the ethanoate has to be half that of the ethanoic acid. When used for cleaning soap solubilizes particles and.
For example sodium hydroxide NaOH aq in its aqueous solutions dissociates as. US 01-145 bottle. ChloricVII acid reacts with sodium hydroxide solution to form a solution of sodium chlorateVII.
The correct structural for mula of butanoic acid is a b c d 19. These acids are a series of organic compounds that contain a carboxyl group COOH and there are a variety of methods that are used to produce them. Making Ethanoic Acid.
Identify two differences betweeh the two titration cur investigation. Because these molecules do not fully dissociate the pH shifts less when near the equivalence point. Technical Grade Sodium Hydroxide 99 Flake 25kgBag Min.
An example of a weak acid is acetic acid ethanoic acid and an example of a weak base is ammonia. KOH CO 2 KHCO 3. Page 6 of 11.
Let us consider the titration ammonium hydroxide against HCl. Vinegar is a solution of a 50 60 acetic acid in alcohol. Bases that you may know about include sodium hydroxide commonly known as caustic soda ammonium hydroxide and ammonia.
100 bottle Contact Now. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Ethanoic acid is the most common example of a carboxylic acid.
Figure 1 Uses of sulfuric acid. Phosphorous acid has a pK a of 200 which makes it stronger than common organic acids like ethanoic acid pK a 476. Some examples of bases are.
By far the largest amount of sulfuric acid is used to make phosphoric acid used in turn to make the phosphate fertilizers calcium dihydrogenphosphate and the ammonium phosphates. It is also used to make ammonium sulfate which is a particularly important fertilizer in sulfur-deficient. In the following example zinc oxide becomes the zincate ion ZnOH 4 as part of soluble sodium zincate when added to concentrated base.
One way of getting this for example would be to mix together 10 cm 3 of 10 mol dm-3 sodium ethanoate solution with 20 cm 3 of 10 mol dm-3 ethanoic acid. Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2. NaOH aq Na aq OH aq The term alkali is often used for water soluble bases.
Ethanol reacts with sodium and for ms two products. Draw and fully label a titration curve from the data collected from the acetic ethanoic acid and sodium hydroxide titration. These ar e a sodium ethanoate and hydr ogen b sodium ethanoate and oxygen c sodium ethoxide and hydr ogen d sodium ethoxide and oxygen 18.
202291 1712 Molarity to Molarity. Acetone acetonitrile ACN benzene ethanol diethyl ether isopropanol methanol methyl ethyl ketone tetrahydrofuran THF. A base is a substance which furnishes hydroxide ions OH when dissolved in water.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. ZnO H 2 SO 4 ZnSO 4 H 2 O. 40 Tons Contact Now.
Ethanoic acid Acetic acid Ethanol. 202291 1701 Molarity to Mass-volume percentage. Due to the hydrolysis of the salt NH 4 Cl formed during the reaction the pH lies in the acid range.
Silica Gel Self Indicator High Quality for Lab Equipment and. Some of these are found in household cleaning products. Create bubbles fog and a colour change adding dry ice to alkaline ammonia or sodium hydroxide solution in this demonstration.
The most common method is oxidising primary alcohols. The main target users are workers and those responsible for occupational safety and health. High Quality Calcium Hydroxide Sale.
Graph the pH versus NaOH added. Vinegar is at least 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements. Uses of sulfuric acid.
Propanone Acetone Propene Propylene Sodium carbonate. Health Hazards of KOH. Sodium hydroxide NaOH 10 1110.
Or 10 cm 3 of 10 mol dm-3 sodium ethanoate solution with 10 cm 3 of 20 mol dm-3 ethanoic acid. In a domestic setting soaps are surfactants usually used for washing bathing and other types of housekeepingIn industrial settings soaps are used as thickeners components of some lubricants and precursors to catalysts. Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products.
Mass-volume percentage to Molarity Recent user inquiry. To react with a base the amphoteric hydroxide often needs to have been freshly produced and the base must be hot and concentrated. The ICSC project is a common undertaking between the World.
Includes kit list and safety instructions. Thus the pH at end point lies in the range of 6 to 4. The addition of hydroxide ions by adding lime sodium hydroxide or potassium hydroxide adjusts the pH because the hydroxide ion reacts with carbon dioxide to form bicarbonate alkalinity.
Methanal and ethanoic acid in this demonstration. Ethanoic acid CH3COOH 36 1045 hydrochloric acid HCl 36 1180. I Sodium hydroxide NaOH or caustic soda used in washing soaps.
This is done in two simplified stages. Includes kit list and safety instructions.
What Salt Is Made When Sodium Hydroxide And Acetic Acid React Together Quora

No comments for "Ethanoic Acid and Sodium Hydroxide"
Post a Comment